How to evaluate temperature excursions with MKT

Jakob Konradsen
USP <1079.2> sets clear rules for evaluating temperature excursions with MKT. Learn 7 key updates for GDP and GMP compliance, what they mean for how you evaluate excursions with MKT, and how to adapt your practices.
A ready-to-use template for documenting excursions with MKT – free to download.

This page explains the key updates in USP <1079.2> and what they mean for pharma and logistics teams managing temperature excursions and overall temperature compliance.
Also see: Temperature excursion investigation guide: How to investigate a excursion faster?
What is USP <1079.2> and why does it matter for temperature excursions?
USP <1079.2> was released on August 1, 2025 by the US Pharmacopeia. It defines how to evaluate excursions using MKT and sets clear expectations for documentation and control.
USP General Chapter <1079.2> provides clear guidance on how to use mean kinetic temperature (MKT) to evaluate temperature excursions in pharma and logistics. For the first time, the chapter makes excursion evaluation explicit – with defined calculation windows, temperature thresholds, documentation requirements, and misuse warnings. This matters if you are responsible for GxP compliance, whether in a warehouse, cross-dock, or global logistics lane.
Also read: Guidelines for temperature mapping in pharmaceuticals
What are the new USP <1079.2> MKT time windows?
One of the most important updates is the definition of precise calculation windows for excursion evaluation. These are based on storage condition type.
Controlled room temperature (CRT)
Calculate MKT over 30 consecutive days, using data from and including the high excursion. For example, if a warehouse experiences a 29 °C spike for three hours, you must calculate MKT over the last 30-day period, including the alarm period.
Controlled cold temperature (CCT)
Calculate MKT over 24 consecutive hours, using data from and including the alarm period. For example, if a refrigerated truck is delayed at customs and warms to 14 °C for six hours, you must calculate MKT for a 24-hour window.
For operations, this means you cannot just average data over a year. Your monitoring system must be able to export the correct intervals – ideally at a maximum of 15‑minute logging.
Why can’t you offset an excursion by cooling down later?
USP <1079.2> warns against misusing MKT. You cannot justify an out‑of‑spec event by showing that the temperature later dropped back within limits. Product degradation is cumulative and not reversible.
For teams, this means no more back‑averaging to smooth out spikes. Each excursion must be evaluated in its own defined window.
Also read: Pharma compliance guide
What excursion limits apply to CRT and CCT in USP <1079.2>?
USP <1079.2> provides specific limits that should be applied when evaluating excursions. These limits supplement product label requirements and stability data.
CRT excursion limits
- Range: 15–30 °C
- Maximum: 40 °C for 24 h
- MKT: Not more than 25 °C over 30 days
CCT excursion limits
- Range: 8–15 °C
- Maximum: 15 °C for 24 h
- MKT: Not more than 8 °C over 24 h
For logistics, this provides a clear reference point for setting alarm thresholds and delays that align with GDP expectations and WHO TRS 961 guidance.
Also see: DOWNLOAD: Temperature excursion investigation template
How should you document and act on excursions?
Why is every excursion a nonconformity under USP <1079.2>?
USP <1079.2> frames every excursion as a nonconforming event. Even if MKT is within limits, it must be documented and justified. The manufacturer always makes the final product disposition decision.
In practice, you should embed a standard record: excursion details, MKT calculation, QA sign‑off, and justification. This ensures your audit trail is intact and GDP‑compliant.
When does an excursion show your system is out of control?
Repeated excursions indicate a system that is not in control. MKT cannot be used to justify poor control. At that point, corrective actions and possibly a CAPA are required.
Logistics teams should define objective triggers. For example, two CCT excursions in a 30‑day period on a lane should trigger a system review. Pharma QA teams should set CAPA triggers that align with GMP expectations.
Also read: Wireless data loggers for pharma monitoring

Download a USP <1079.2> excursion evaluation checklist
Download the free template to document excursions with MKT, complete with decision flow and evaluation table.
Does USP <1079.2> apply to pharma and logistics companies?
The scope covers all handlers of finished drug products, except the patient. That means manufacturers, distributors, 3PLs, storage hotels, wholesalers, pharmacies, and hospitals.
For pharma and logistics teams, this means every warehouse, cross‑dock, truck, and storage unit is in scope. Harmonizing SOPs across sites is critical to GDP, GMP, and USP compliance.
Also read: GDP regulations for pharmaceutical storage and transport
How do mapping, monitoring, and alarms support USP <1079.2> compliance?
USP <1079.2> references continuous monitoring and event‑based evaluation. Temperature mapping informs where to place sensors. Monitoring provides the data. Alarms surface excursions in time to act.
For operations, this means aligning your mapping outputs, monitoring intervals, alarm delays, and thresholds directly with USP excursion logic, GDP, and WHO TRS 961 best practices.
Also read: Pharma calibration services
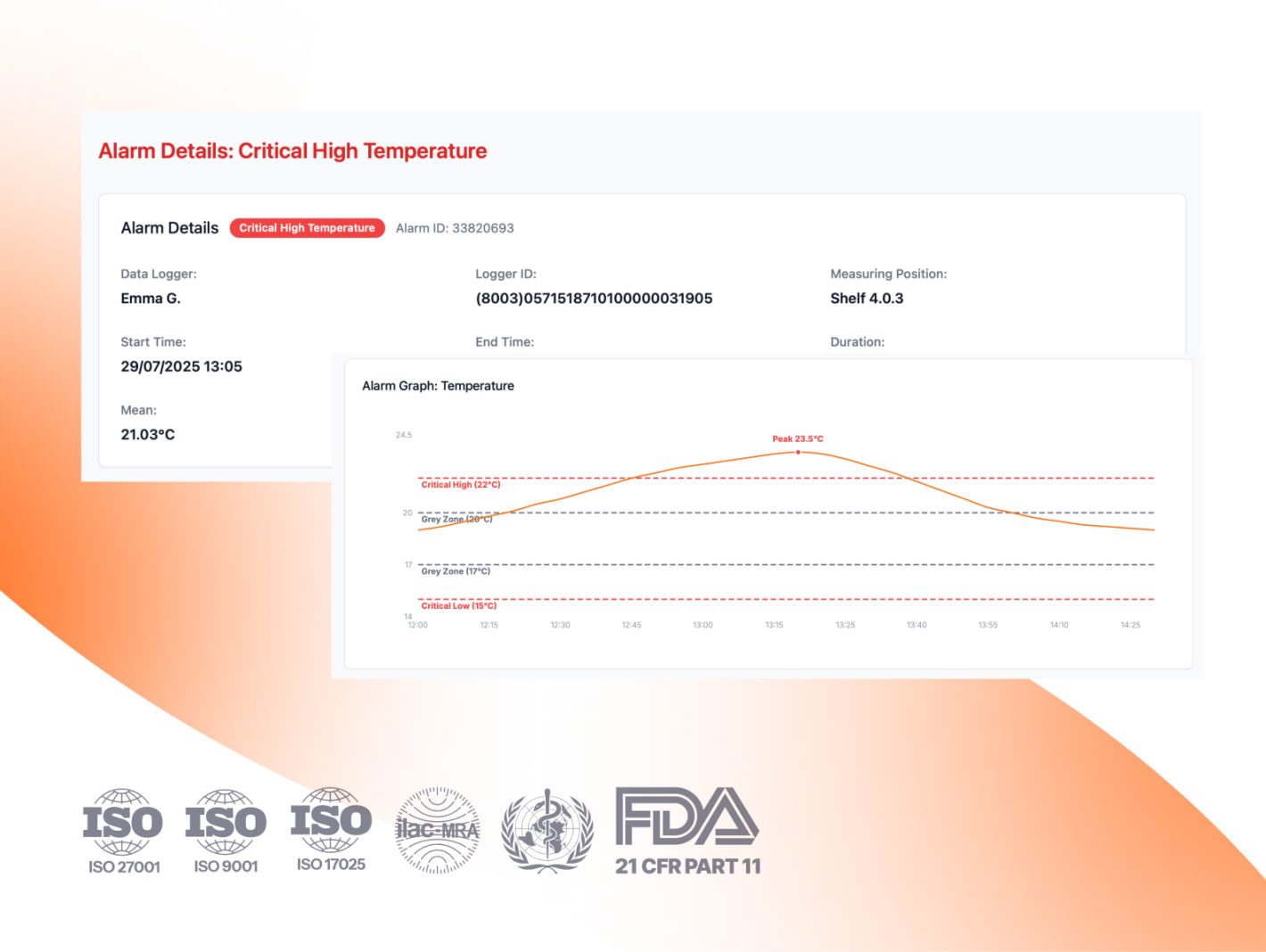
How Eupry’s new alarm handling provides faster, safer excursion evaluation
Eupry’s alarm handling system for temperature monitoring puts USP <1079.2> into practice with compliant thresholds, validated delays, and an audit-ready trail, as well as providing better incident handling functionalities.