Continuous aircraft mapping (and monitoring)
Turn your aircraft into a continuously validated cold chain environment.
- Data within 2 minutes of landing: Auto-generated compliance reports verify aircraft conditions and inform decisions.
- Prevent product losses with data: Adapt ground transport when temperature limits are approached with instant data.
- Reduce costs by 20-30%: Lower compliance costs and validate ULD strategies that cut fuel and emissions.

Trusted by 1000+ GxP companies worldwide, including:
The million-dollar “aircraft blind spot”
Your warehouses are mapped. Your trucks are monitored. But your aircraft cargo holds? Still a compliance gap that forces overcompensation and product loss.
Data arrives days after delivery
Shipment-level monitoring provides data only after products are delivered, creating holding costs and quality risks of product losses.
Delayed intervention = risks to products
No flight data until delivery means problems are discovered when corrective action is too late, and you can't adjust to prevent losses.
Costly overcompensation
Without aircraft environmental data, you are forced to overinvest in active ULDs ($20-50k vs. $2-5k passive) but don't know if it is necessary.
Unnecessary environmental impact
Active ULDs weigh 600 kg more than passive containers, generating avoidable emissions because you can't validate passive alternatives.
Turn your aircraft into a pharma-approved, temperature-controlled route with Eupry
Continuous mapping and monitoring transforms pharmaceutical aircrafts into continuously validated cold chain environments with automatic documentation.
- Full visibility with real-time monitoring from takeoff to landing
- Comprehensive mapping data just two minutes after touchdown
As the world’s only FAA-approved loggers for permanent installation, Eupry syncs seamlessly with FlightRadar to provide precise, end-to-end tracking for your most sensitive cargo.
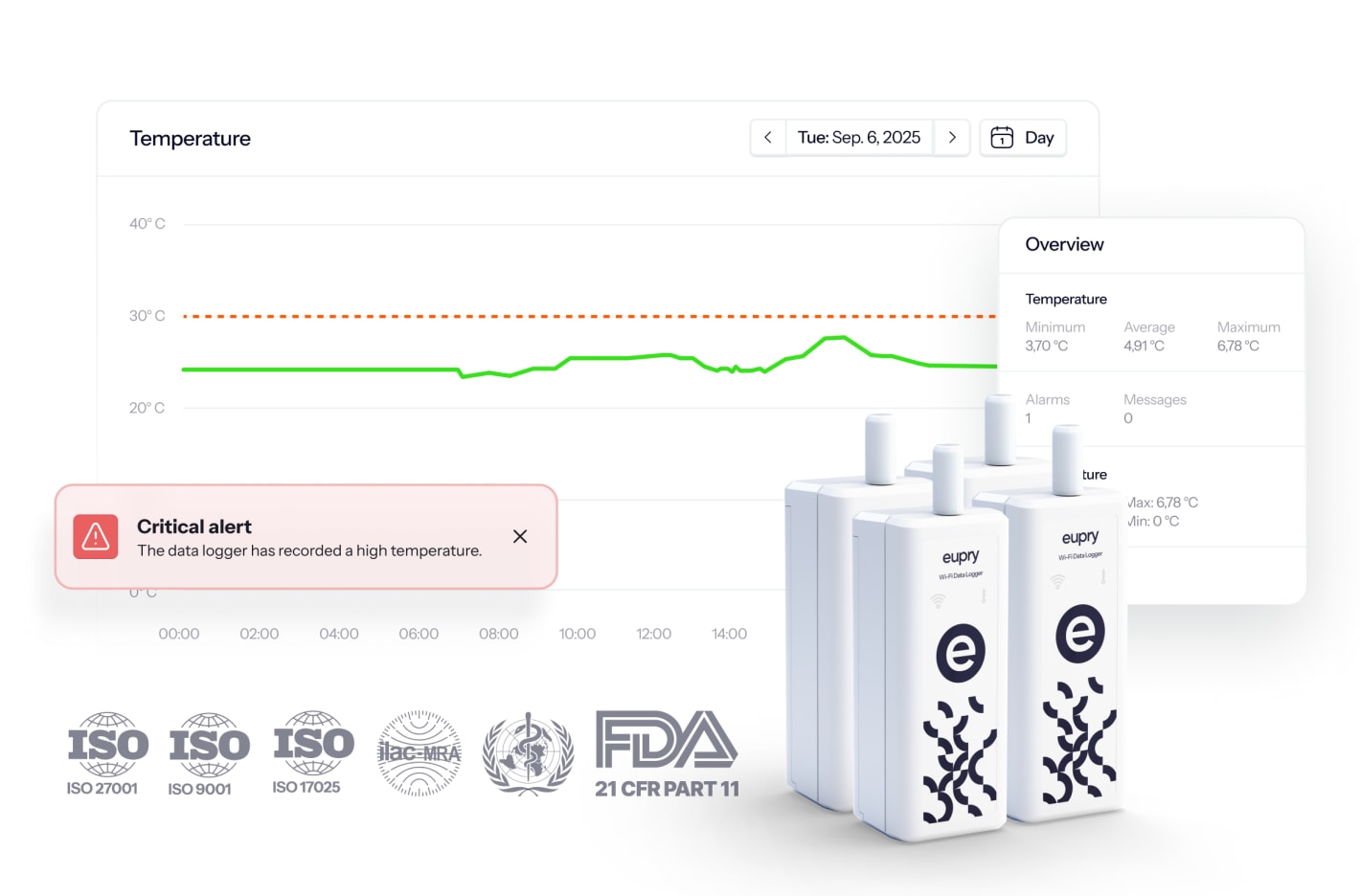
Verify conditions in minutes
Assesses conditions instantly with all flight data available within 2 minutes of landing instead of waiting for final delivery.
Eliminate product loss
Instant insights into flight data makes it possible to adjust ground transport to adjust stability budgets and protect products.
Lower costs by 20-30%
Consolidate vendors to reduce costs by 20-30%. Validate passive ULD use to cut up to 19 t per flight, lowering fuel costs and CO₂.
Full cold chain coverage
Monitor your entire cold chain in ONE solution – from warehouse to aircraft and delivery – with Eupry's end-to-end solution.
Approved by The Federal Aviation Administration
Eupry's CMM solution for is approved by The Federal Aviation Administration (FAA) - and designed for the needs of GxP.


Get in touch
Need more information or a tailored price? Fill out the form, and we will be in touch asap!
How Eurpy's continuous mapping and monitoring for aircrafts works
Initial mapping establishes your baseline
- Our team conducts mapping across cargo holds with FAA-approved sensors during your operational flights.
- You receive statistical proof of environmental performance - the foundation for passive ULD validation and customer contracts.
No empty aircraft, no schedule disruptions.

Permanent CMM maintains continuous validation
- Permanent sensors upload data within 2 minutes of landing
- You act on flight data instantly to prevent product losses
- Eupry handles all technical maintenance and calibration
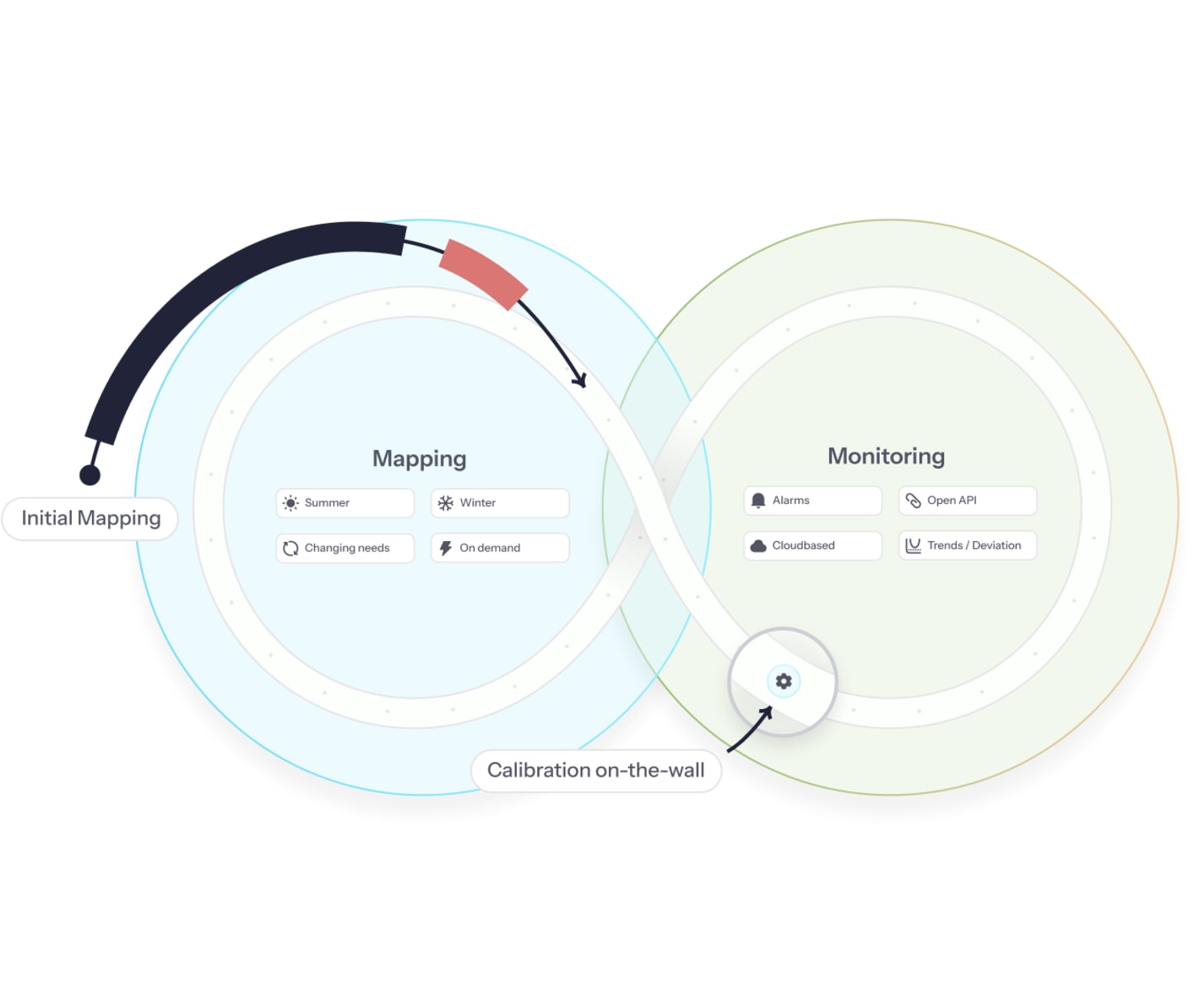
Act proactively, prevent product losses
Our platform triggers real-time alerts when temperature limits approach, and a full flight data report - with FlightRadar integration - is available within 2 minutes of landing.
- Review temperature profiles and assesses conditions.
- If limits are approcahed, adjust ground transport.
Shift from discovering problems after delivery to preventing them.
= No more product losses.

One solution, all your cold chain data
Unify aircraft CMM with warehouses, fleet, and every touchpoint – one vendor, one source of truth.
- Live alerts protect products in transit
- Automated data flow eliminates manual work
- AI analytics detect anomalies and forecast delays
Traditional approach vs. aircraft CMM
What changes with continuous mapping and monitoring for your aircraft?


Download a product catalog
Get an instant overview of how the solutions work.
FAQ about getting started with aircraft CMM
Get started with CMM for aircrafts
Release in minutes, prevent losses, and lower costs by 20-30%.
Learn more about aircraft thermal compliance

Aircraft temperature mapping: Solution for GDP
Learn how mapping of pharmaceutical aircrafts can unlock smarter ULD use and lane qualification.

Free download: Aircraft temperature mapping protocol template
Get a step-by-step protocol to help you plan and execute GDP aircraft temperature mapping.
Temperature monitoring: Guidelines for pharma air freight
Get the complete guide to pharmaceutical air cargo temperature monitoring, covering GDP and IATA.





